
Alopecia areata is a common hair loss condition that is characterized by acute onset of non-scarring hair loss in usually sharply defined areas ranging from small patches to extensive or less frequently diffuse involvement. Depending on its acuity and extent, hair loss is an important cause of anxiety and disability. The current understanding is that the condition represents an organ-specific autoimmune disease of the hair follicle with a genetic background. Genome-wide association studies provide evidence for the involvement of both innate and acquired immunity in the pathogenesis, and mechanistic studies in mouse models of alopecia areata have specifically implicated an IFN-γ-driven immune response, including IFNγ, IFNγ-induced chemokines and cytotoxic CD8 T cells as the main drivers of disease pathogenesis. A meta-analysis of published trials on treatment of alopecia areata states that only few treatments have been well evaluated in randomized trials. Nevertheless, depending on patient age, affected surface area and disease duration, an empiric treatment algorithm can be designed with corticosteroids and topical immunotherapy remaining the mainstay of therapy. The obviously limited success of evidence-based therapies points to a more important complexity of hair loss. At the same time, the complexity of pathogenesis offers opportunities for the development of novel targeted therapies. New treatment opportunities based on the results of genome-wide association studies that implicate T cell and natural killer cell activation pathways are paving the way to new approaches in future clinical trials. Currently, there are ongoing studies with the CTLA4-Ig fusion protein abatacept, anti-IL15Rβ monoclonal antibodies and the Janus kinase inhibitors tofacitinib, ruxolitinib and baricitinib. Ultimately, the options available for adapting to the disease rather than treating it in an effort to cure may also be taken into consideration in selected cases of long-standing or recurrent small spot disease.
Avoid common mistakes on your manuscript.
Alopecia areata (AA) is a common hair loss condition with a lifetime prevalence of approximately 2% [1] that is characterized by acute onset of non-scarring hair loss in usually sharply defined areas [2]. Some patients lose hair in only a small patch, while others have more extensive or less frequently diffuse involvement [3]. Any hair-bearing area can be affected, but the most noticeable are the scalp, the beard area and the eyebrows.
Hair is a power symbol, as the Biblical Samson teaches us in Judges 16. Hair falling out in dreams is interpreted as loss of power and control. The hair loss dream is typically a manifestation of a stressful situation. Ultimately, hair dreams represent a situation that is beyond one’s control. The same type of helpless feeling is experienced in waking life when hair is suddenly lost.
AA has obviously existed at all times, and is referred to by Roman encyclopaedist Aulus Cornelius Celsus (25 bc –50 ad ) in his celebrated treatise on medicine (“De Medicina”) in the chapter “De capillis fluentibus”, or can be spotted in great works of art such as in Francisco Goya’s (1774–1828) “Il Matrimonio” in the Museo del Prado in Madrid, Spain.
Depending on its acuity and extent, hair loss is obviously an important cause of anxiety and disability. At any given time, AA is found in 0.1 to 0.2% of the population [4], with all ethnic backgrounds appearing to be similarly affected, and no difference between the sexes [1]. The onset of AA is before the age of 40 years in 70–80% of those affected; however, a significant proportion (48%) will present with the condition during their first and second decades, making AA the most common cause of hair loss in otherwise healthy children [5]. General practitioners are often called upon as primary care takers, but often are not familiar with the condition and may fail to provide patients with the appropriate management or to refer them to the dermatologist.
The most frequent clinical presentation of AA is in single or multiple patches. The characteristic patch of AA is usually round or oval, and is completely bald and smooth. Occasionally, AA may progress to complete baldness, which is referred to as alopecia (areata) totalis (AT). When the entire body suffers from complete hair loss, it is referred to as alopecia (areata) universalis (AU). Ophiasis is a form of AA characterized by the loss of hair in the shape of a wave at the circumference of the head (Fig. 1), and was originally referred to by Celsus. Its name derives from the ancient Greek word for snake (“ophis”), because of the snake-like shape of the pattern of hair loss.
The skin within the patches is inconspicuous, and hair follicles are intact. Toward the periphery of the area of active hair loss, one can usually note characteristic, smaller 3- to 4-mm exclamation mark hairs and/or black dots (Fig. 2a). They basically represent dystrophic hairs that have fractured tips due to an inhibition of cell division in the hair matrix at the level of the hair bulb. Severe inhibition leads to fracturing of the hair shaft before emergence from the scalp and then appears as a black dot (or cadaverized hair). The exclamation mark appearance describes a damaged strand of hair that is thicker at its distal end and notably thinner proximally as it enters the scalp. Exclamation mark hairs typically are found in acute forms of AA, and are not seen in patients with long-standing areas of hair loss. The latter rather presents with yellow dots within the affected scalp. Visualized at a higher magnification (×10) than the naked eye with help of a simple handheld dermatoscope (Heine Delta®, DermoGenius®, DermLite PRO HR® or DermLite DL3®), they present as quite monomorphous, though variably sized, yellow, round dots that are devoid of hair or contain vellus hair (Fig. 2b). They represent dilated follicular infundibula that contain sebaceous and keratinous material [6].
Hair recovery in AA usually occurs as a uniform process with thin white hair emerging first, followed by healthy pigmented hair. At times, it may occur in a concentric targetoid hair re-growth pattern [7], or in tufts of white hair [8].
In 2002, Sato-Kawamura et al. [21, 22] suggested naming a peculiar type of inflammatory non-cicatricial alopecia that is characterized by marked female predominance and a uniquely short clinical course acute diffuse and total alopecia of the female scalp. It is basically identical with a subtype of AA presenting with diffuse hair loss as originally proposed in the German literature by Braun-Falco and Zaun 40 years earlier in 1962 [23]. Diffuse alopecia areata or alopecia areata incognita, yet another synonymous designation proposed by Rebora in 1987 [24], represents an uncommon variety of AA characterized by diffuse hair shedding in the absence of typical patches.
Marie Antoinette syndrome designates the condition in which scalp hair suddenly turns white [25]. The name alludes to the French Queen Marie Antoinette (1755–1793), whose hair allegedly turned white the night before her last walk to the guillotine during the French Revolution. Although the actual incidence is rare, this stigmatizing phenomenon has captured the storytellers’ imagination like few other afflictions as a sign of grave sorrow. The first documented case of sudden hair whitening can be traced back to the Talmud (83 ad ), which describes a scholar who was appointed chief of the main Israeli Talmudic academy at the young age of 17 years. Upon his wife’s concern that he looked too youthful for the position, he developed 18 rows of white hair [26].
History also records that the hair of the English martyr Sir Thomas More (1478–1535) turned white overnight in the Tower of London before his execution [27]. Thomas More was beheaded in 1535 after having refused to sign the Act of Supremacy that declared King Henry VIII of England Supreme Head of the Church of England over the Pope. More modern accounts refer to the turning white of hair in survivors of bomb attacks during World War II [28, 29].
Although Marie Antoinette was originally chosen as eponym for the syndrome, Thomas More who turned white in 1535 ought to have the right of seniority over the Queen of France who succumbed to the same fate only in 1793. Since there seems to be no other particular reason for favouring Marie Antoinette over Thomas More, it seems logical to use the term Marie Antoinette syndrome for the condition afflicting women and Thomas More syndrome for men.
Today, the syndrome is interpreted as an acute episode of diffuse AA in which the very sudden whitening of hair is caused by the preferential loss of pigmented hairs. As early as 1901, immunology research pioneer and Nobel Prize Laureate (1908) in Medicine for his work on phagocytosis, Elie Metchnikoff (1845–1916), believed that whitening of hair was related to pigment absorbed and carried away by phagocytes that he observed in the vicinity of dystrophic hair follicles and called pigmentophages [30]. In 1981, Guin et al. [31] reported a lady whose hair turned white over 3 months with hair thinning but no patches of alopecia. Immunofluorescence studies demonstrated immune deposits along the follicular epithelium, suggesting an underlying immunological mechanism. Finally, case reports of sudden whitening of hair in association with easily plucked hairs, exclamation mark hairs and patchy alopecia typical for AA, and in co-existence with other autoimmune phenomena, such as vitiligo, supported the immune theory [32]. Ultimately, these observations have led some authorities to speculate that the autoimmune target in AA may be related to the hair follicle melanin pigment system [33]. McBride and Bergfeld [34] described mosaic hair colour changes in two patients with extensive AA, and suggested that these processes may result from localized immunological reactions against hair follicle melanocytes in a multifocal pattern with subsequent changes in hair colour. In contrast to total whitening of hair, this mechanism would account better for the pattern of 18 rows of white hair originally described in the Talmud.
Although early accounts of overnight whitening of hair are substantiated by more recent case reports in the scientific literature, it may be suspected that the phenomenon has been used both as a literary stylistic means in fiction, with the aim of dramatizing, and in historical accounts with the claim of authenticity [35]. Scrutinizing the case of Henry III of Navarra (later King Henry IV of France), whose hair allegedly turned white overnight on the occasion of the horrors of St. Bartholomew’s Day massacre in 1572, Navarini and Trüeb [36] found inconsistencies between Henry’s biography and the current pathophysiological understanding of the condition. Since a pre-requisite for overnight whitening of hair would be that the individual in question has a salt-and-pepper pattern with pigmented hairs interspersed with white hairs and the pigmented hairs selectively shed while the white hairs remain, it seems quite improbable that Henry III of Navarre’s hair should have turned white so rapidly, as he was only 19 years old at the time in question.
Today, AA is considered an organ-specific autoimmune disease of the hair follicle with a genetic background.
Genetic factors have been implicated with the reports of familial incidence of AA in a range between 10 and 27% [37,38,39]. Among first-degree relatives of 348 severely affected patients, van der Steen et al. [40] found that one of the parents was affected in 7%. Among the sibs, 3% had AA, while AA was present in 2% of the children. Ultimately, Martinez-Mir et al. [41] suggested that AA fits the pattern of a complex genetic trait based on its prevalence in the population of approximately 2%, a tenfold increased risk for first-degree relatives of affected individuals with no clear Mendelian pattern of inheritance within affected families, a concordance in monozygotic twins of 55% [42] and a Gaussian distribution of severity that ranges from patchy localized hair loss on the scalp to the complete absence of hair on the body. With regard to the genetic susceptibility to AA and the disease severity, Price and Colombe [43] originally suggested several genes associated with the human leukocyte antigen (HLA) class II alleles: HLA-DQ3 appeared to be the general susceptibility allele for AA, while patients with long-standing disease patterns, namely long-term patchy AA and long-term AT or AU, could be differentiated by their particular HLA associations with HLA antigens DR4, DR11 and DQ7.
The hair follicle is an immune-privileged site, with low levels of major histocompatibility complex (MHC) expression [44]. It is assumed that AA results from a breakdown in immune privilege with the subsequent assault on the follicle on the level of the bulb by CD8 + T lymphocytes [45]. T lymphocytes have been shown to be oligoclonal and autoreactive [46], and are predominantly present in the peribulbar inflammatory infiltrate. The peribulbar lymphocytic infiltrate induces hair follicle keratinocytes to undergo apoptosis that results in inhibition of cell division within the hair matrix and of hair shaft production. Depending on the severity of inflammation and apoptosis, the net result is a telogen-type effluvium and/or dystrophic anagen effluvium.
Notably, AA occurs in association with other autoimmune diseases, such as autoimmune thyroid disease, in between 8 and 27% [37, 47,48,49,50], and vitiligo in between 4 and 9% [2, 47, 51, 52] depending on author, and with respective circulating anti-thyroid and anti-parietal cell autoantibodies [50, 53, 54]. Finally, autoantibodies to follicular components have been detected. Tobin et al. [55] tested 39 AA sera and 27 control sera by Western immunoblotting for antibodies to 6 M urea-extractable proteins of normal anagen scalp hair follicles. At serum diluted 1:80, all AA subjects (100%), while only 44% of the control individuals had antibodies directed to one or more antigens of approximately 57, 52, 50, 47 or 44 kD. The incidence of antibodies to individual hair follicle antigens in AA was up to seven times more frequent than in control sera and their level up to 13 times greater and was statistically significant for all five antigens. Tissue specificity analysis indicated that these antigens were selectively expressed in hair follicles. Candidate autoantigens that have been identified include the 44/46 kDa hair-specific keratin (expressed in the precortical zone of anagen hair follicles) and trichohyalin (an important intermediate filament-associated protein) expressed in the inner root sheath of the growing hair follicle [56].
Ultimately, studies have demonstrated that AA scalp skin grafted on to nude mice with severe combined immunodeficiency grow hair and that infiltrating lymphocytes in the graft are lost, while it is possible to induce AA in human scalp explants on these mice by injecting T lymphocytes with scalp homogenate [57].
The C3H/HeJ mouse is an inbred laboratory strain that spontaneously develops an adult-onset disease that resembles adult-onset alopecia areata in humans. Sundberg et al. [58] identified 4 genetic susceptibility loci on mouse chromosomes 8, 9, 15 and 17, wherein the chromosome 17 locus corresponds to HLA orthologs. C3H/HeJ mice with hair loss have been shown to have circulating antibodies to hair follicles similar to those present in humans with AA [59], corroborating that these mice represent a fitting model for human AA, and supporting the hypothesis that AA results from an abnormal autoimmune response to the hair follicle [59]. However, to what extent B cells are involved in the pathogenesis of AA, and whether antibodies to hair follicle-specific proteins [56] contribute to disease or only represent an autoimmune epiphenomenon, remains to be elucidated.
Increased reactive oxygen species and high cellular stress level have been identified in AA [60,61,62], and studied in its relationship with disease severity, duration, recurrence and pattern [63]. Antioxidant/oxidant balance perturbation is a common feature in autoimmune, emotional and environmental stress. Whether these changes play a role in pathogenesis of AA or again only result from the inflammatory process requires further investigation.
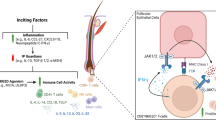
Genome-wide association studies performed by Petukhova et al. [64] demonstrated an association with genomic regions containing several genes controlling the activation and proliferation of regulatory T cells, cytotoxic T lymphocyte-associated antigen 4 (CTLA4), interleukin (IL)-2/IL-21, IL-2 receptor A (IL-2RA; CD25) and Eos (also known as Ikaros family zinc finger 4 (IKZF4)), as well as the HLA region. Furthermore, they found a region of strong association within the cytomegalovirus UL16 binding protein (ULBP) gene cluster on chromosome 6q25.1, encoding activating ligands of the natural killer cell receptor NKG2D. By probing the role of ULBP-3 in disease pathogenesis, they showed that its expression in lesional scalp from patients with AA was markedly upregulated in the hair follicle dermal sheath during active disease. This study provides evidence for the involvement of both innate and acquired immunity in the pathogenesis of AA. Furthermore, the authors have defined the genetic underpinnings of AA, placing it within the context of shared pathways among autoimmune diseases, and implicating a novel disease mechanism, the upregulation of ULBP ligands, in triggering autoimmunity.
The possibility that cytomegalovirus (CMV) may be involved in the pathogenesis of AA was originally proposed by Skinner et al. [65] after detection of CMV DNA sequences using polymerase chain reaction in scalp biopsies of AA patients; however, this hypothesis was not confirmed by subsequent authors [66,67,68].
A recent genome-wide meta-analysis in AA resolved the HLA associations and revealed two new susceptibility loci. Betz et al. [69] performed a meta-analysis of research on AA by combining data from their genome-wide association studies, and replication with supplemented ImmunoChip data for a total of 3253 cases and 7543 controls. The strongest region of association is the MHC, where they fine mapped four independent effects, all implicating HLA-DR as a key etiologic driver. Outside the MHC, they identified two novel loci that exceed the threshold of statistical significance. Candidate susceptibility gene expression analysis in these regions demonstrated expression in relevant immune cells and the hair follicle. These findings uncover new molecular pathways disrupted in AA, including autophagy/apoptosis, transforming growth factor beta/Tregs and Janus kinase (JAK) signalling.
Notably, mechanistic studies in the mouse models of AA have specifically implicated an interferon (IFN)γ-driven immune response, including IFNγ, IFNγ-induced chemokines and cytotoxic CD8 T cells as the main drivers of disease pathogenesis [70].
Finally, Fischer et al. [71] investigated the contribution of copy number variants (CNVs) to AA, a prominent class of genomic variants involved in autoimmune disorders. Again, a genome-wide and a candidate gene focused CNV analysis were performed in a cohort of 585 AA patients and 1340 controls of Central European origin. A nominally significant association with AA was found in the genes MCHR2 and MCHR2-AS1, implicated in melanin-concentrating hormone (MCH) signalling. This might indicate a relationship between AA, pigmentation and MCH signalling, consistent with the originally perplexing phenomena of 18 rows of white hair growing in the Talmud scholar, the Marie Antoinette and Thomas More syndromes, and, in general, why the hair of AA patients frequently shows a change in colour to white upon re-growing after an acute episode of AA.
For centuries, physicians propagated the viability of a complex approach in the diagnosis and treatment of disease, while today, we stress on specification. This brought up the question of how to wholly evaluate the state of an individual who suffers from a number of conditions simultaneously. Ultimately, Alvan R. Feinstein came out in 1970 with the concept of co-morbidity, which has been defined as presence of one or more additional diseases co-occurring with a primary disease; or the effect of such additional diseases, whereby the additional disorder may also be a behavioural or mental disorder [72].
The interrelations of disease, age and drug pathomorphism greatly affect the clinical presentation and progress of the primary nosology, character and severity of complications, impair the patient’s life quality and impede the diagnostic and remedial process. The presence of co-morbidity must therefore be taken into account when selecting the algorithm of diagnosis and treatment plans for any given, including trichological disease. Ultimately, the dermatologist participates with the other medical disciplines in the diagnosis and treatment of all types of hair problems as they relate to systemic disease.
While AA occurs in approximately 0.1–0.2% of the general population, it is found in approximately 9% of the patients with Down syndrome [73].
Since AA represents an autoimmune disease, it is not surprising that an association exists with other autoimmune conditions [47,48,49,50,51,52,53,54]. Moreover, more recently additional co-morbid conditions have been identified that may have a disease-modifying effect and therefore must be included in the treatment plan (Table 1).
The art of using corticosteroids for treatment of AA is maximizing efficacy and minimizing toxicity.
Single patches of AA are best treated with intralesional triamcinolone acetonide (ITA) in a concentration between 2.5 (eyebrows and beard area) and a maximum of 10 mg/ml (scalp), depending on the localization, by jet injector [135] or insulin syringe (frontal and temporal regions, eyebrows, beard area) at 4–6-week intervals, as long as needed (Fig. 5a–c). ITA has proven to have a better efficacy in the treatment of localized AA than topical treatment, either with betamethasone valerate [136, 137] or with tacrolimus [137]. Since patchy AA is the most prevalent form of the disease, and ITA is the most frequently practiced treatment for this condition, Samrao et al. [138] conducted a study on patients with AA treated in this way for at least 20 months to evaluate the effect on bone mineral density using dual-energy x-ray absorptiometry (DXA). Fifty percent of the patients had abnormal DXA results. Patients with the following risk factors were more likely to be affected: age older than 50 years, body mass index less than 18.5 kg/m 2 , lack of weight-bearing exercise, smoking history, postmenopausal status, past stress fracture, family history of osteopenia or osteoporosis and a cumulative ITA dose of greater than 500 mg.
In an attempt to circumvent side effects related to the use of corticosteroids, Chu et al. [139] evaluated the benefit of different concentrations (2.5, 5 and 10 mg/ml) of ITA in AA, and did not find any difference in re-growth of hair between the different concentrations, enabling injection of larger surfaces at lower concentrations and lesser cumulative doses of triamcinolone acetonide.
Acute and widespread AA (>30% surface area) are best treated with systemic corticosteroid therapy, either orally or intravenously (Fig. 6a, b). Again, in an attempt to circumvent side effects, pulsed administration has been proposed for treatment of AA: Sharma [140] originally proposed 300 mg oral prednisolone pulses at 4-week intervals, for a minimum of four doses or until cosmetically acceptable hair growth was obtained. 58.3% of the patients with widespread AA showed cosmetically acceptable hair growth. Response was evident on average after 2 to 3 months of therapy. In a subsequent study, Sharma and Muralidhar [141] reported efficacy and safety of monthly oral corticosteroid pulse therapy also for treatment of young patients (up to 18 years of age), including children. Children aged less than 12 years received betamethasone sodium phosphate as soluble equivalent to prednisolone 5 mg/kg body weight every month. Side effects were minimal and included transient giddiness, headache and epigastric burning. Subsequently, Kar et al. [142] confirmed in the first placebo-controlled study the usefulness of oral prednisolone pulse therapy in AA. Agarwal et al. [143] alternatively suggested twice weekly 5 mg betamethasone oral pulse therapy on two consecutive days per week for a total duration of 12 weeks.
To determine the effectiveness of intravenous pulse therapy, much in the same manner as for treatment of other autoimmune diseases, Friedli et al. [144] originally performed an open prospective study of patients with rapid and extensive hair loss (>30% scalp area) for less than 1 year (first occurrence or relapse). Two hundred fifty-milligramme intravenous methylprednisolone (IV-MPPT) was administered twice a day on three consecutive days. A single series of IV-MPPT was well tolerated and appeared to be effective in patients with rapidly progressing extensive multifocal AA, but not those with ophiasic and AU. Subsequently, Nakajima [145] confirmed the efficacy of IV-MPPT in a larger study of patients aged >15 years with AA. With IV-MPPT (500 mg methylprednisolone on three consecutive days, in 3 cycles, 4 weeks apart) within 6 months of disease onset, remission rates were 88% for multilocular AA with surface area 50% and 21.4% in AT/U. Performed after 6 months of disease onset, the remission rate decreased to 15.8%. Im et al. [146] studied the outcome and prognostic factors in patients with severe AA treated with IV-MPPT on three consecutive days. All of the patients had rapid and extensive hair loss with the bald area exceeding 50% of the scalp. Seventy percent showed terminal hair growth, and 41.4% complete recovery with acceptable cosmetic results. The prognostic factors that influenced successful outcome of IV-MPPT for AA were disease duration before treatment in relation to the type of AA. A good response was obtained for all types of AA with a duration of 3 months or less before treatment, and for the multifocal type of AA, with a duration of
Smith et al. [148] investigated the outcome of IV-MPPT in severe childhood AA with short disease duration. Five patients had AT/U and 13 had multilocular AA. All patients underwent 2 or 3 cycles of IV-MPPT at monthly intervals (maximum 500 mg/day on three consecutive days). Within 7 months of last IV-MPPT session, 10 of 18 children had good response, with eight showing improvement within the first 4 months. Seven of the initial 10 good responders experienced relapses, with an estimated median time to relapse of 8 months. Therefore, even early in the course of disease, IV-MPPT did not convincingly affect the long-term outcome of severe AA in the children age group. IV-MPPT was generally well tolerated. One patient experienced transient mood changes during the first cycle; three patients complained of a metallic taste during the infusions. Acne vulgaris was aggravated in two teenage patients.
For treatment of widespread and long-standing (severe and refractory) AA, Tosti et al. [149] evaluated topical clobetasol. With 0.05% topical clobetasol ointment under occlusion (Saran wrap) on six consecutive nights per week, over 6 months re-growth of hair was achieved in AT/U in 17.8% (Fig. 7a, b). Negative prognostic features for treatment success were positive family history for AA, first manifestation of disease before the age of 10 years and association with atopic disease or autoimmune thyroid disease. Folliculitis and acne were the frequent side effects. Despite positive clinical results obtained using clobetasol ointment with occlusive dressing, this approach has a low patient compliance, especially in patients with residual hair. Therefore, in a subsequent study performed again by Tosti et al. [150], 0.05% clobetasol foam was applied twice daily on five consecutive days per week and proved to also be effective, safe and well tolerated, with good cosmetic acceptance and patient compliance (Fig. 8a, b). Folliculitis occurred in a smaller proportion of patients. No significant modifications in cortisol and ACTH blood levels were observed.
Topical immunotherapy with allergic contact sensitizers has been used from the 1970s to treat dermatological conditions assumed to result from an altered immunological state. Dinitrochlorobenzene (DNCB) is a synthetic compound that works as a haptene by combining with proteins to become a complete antigen resulting in an allergic contact dermatitis upon sensitization, and has predominantly been used for the study of experimental allergic contact dermatitis in animals. The use of DNCB to treat extensive AA was originally reported to be successful in 1976 based on two cases in whom half of the bare scalp was treated successfully [151]. The underlying mode of action is hypothesized to be related to factors inherent to the late phase of allergic contact dermatitis. These supposedly modulate the T cell-mediated mechanisms underlying AA, including involvement of cytokines and growth factors, such as IL1 beta [152], while the therapeutic effect mediated by contact sensitizers is assumed to be mediated by counteracting pro-inflammatory cytokines such as TNF-alpha, IL-10 or transforming growth factor (TGF)-beta 1 [153].
Due to mutagenicity in the Ames test, DNCB was substituted with squaric acid dibutyl ester (SADBE) by Happle et al. [154] who originally tested the compound dissolved in acetone and applied weekly to one side of the head, the other side serving as control. In 46 of 53 patients with extensive or total AA treated in this manner (87%), hair re-grew either exclusively on the treated side or considerably faster and denser on this side. In some patients, continuous treatment failed to maintain the response. Persistent response was observed in 37 patients (70%).
At present, yet another contact sensitizer, diphenylcyclopropenone (DPCP), is considered as the agent of choice for topical immunotherapy of AA [155]. DPCP is more stable in acetone and is relatively cheaper than SADBE [156]. Nevertheless, in a comparative study of SADBE and DPCP for the treatment of AA, SABDE proved to be more efficacious and to have lower frequencies of side effects than DPCP [157]. Again, Happle et al. [158] introduced the use of DPCP for topical immunotherapy of AA, and their positive results were soon confirmed by other authors [159, 160]. In a retrospective study of 68 patients with severe AA (>40% scalp hair loss) treated for at least 5 months with topical DPCP, Pericin and Trüeb [161] found an overall response rate of 70.6% with 30.9% complete remission and 39.7% partial remission (Fig. 9a–c). Among the investigated prognostic factors for the outcome of DPCP therapy, the extent of hair loss before therapy was the main predictor for therapeutic success: total remission rates for multilocular AA were 43.8%, for subtotal AA and ophiasis 33.3% and for AT/U 21.4%, irrespective of disease duration. A long period of therapy is required and may increase the percentage of responders, especially in AT/U. Finally, DPCP therapy is associated with a high relapse rate. Therefore, maintenance therapy is recommended to reduce the risk of relapse. Subsequent authors confirmed that topical immunotherapy with DPCP is effective for treatment of widespread and long-standing AA, and is usually well tolerated [162,163,164,165,166,167,168,169,170]. Its efficacy has also been demonstrated in children [171, 172]. Addition of oral fexofenadine, an H1-receptor antagonist, may partially inhibit the itch of contact dermatitis induced by DCP in patients with alopecia areata [173]. Moreover, Inui et al. [174] suggested that oral fexofenadine may enhance the efficacy of contact immunotherapy for extensive AA in patients with an atopic background. Poor accessibility, high cost of the compound and poor patient compliance are major drawbacks of topical immunotherapy of AA.
In an analogy to other lymphocyte-mediated autoimmune diseases, Joly [175] proposed the use of methotrexate (MTX) alone or in combination with low-dose oral corticosteroids for the treatment of AT/U with an overall success rate of 64%. Best results are achieved with subcutaneous MTX at the maximal dosage of 30 mg weekly in combination with 20 mg oral prednisone daily: re-growth of hair begins within 2 to 4 months of this regimen (Fig. 10a–f). Lasting improvement required continuous treatment in most cases, though with a significant lower dosage of MTX after gradually tapering the oral corticosteroid, once hair re-growth has set in [176]. Ultimately, drug toxicities are to be carefully weighed out against treatment benefit, and therapeutic drug monitoring following the respective guidelines is strongly recommended.
The original report of successful treatment of a case of AU with subcutaneous efalizumab by Kaelin et al. [177] could subsequently not be confirmed by a 3- to 6-month placebo-controlled trial with efalizumab in a cohort of 62 patients with moderate-to-severe AA [178]. Efalizumab is a recombinant humanized monoclonal antibody designed to treat autoimmune diseases by binding to the CD11a subunit of lymphocyte function-associated antigen 1 and inhibiting lymphocyte activation and cell migration out of blood vessels into tissues. Efalizumab was associated with fatal brain infections [179] and therefore withdrawn from the market in 2009.
Namazi [180] originally proposed the use of statins for treatment of a variety of dermatologic conditions characterized by ingress of activated leucocytes into the skin, including AA. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitors (statins) are currently used for reducing atherogenesis and cardiovascular morbidity, but there is increasing evidence that they may also have immunomodulatory activities. Robins [181] reported the original indicator case of hair re-growth in a patient with AU following initiation of simvastatin and ezetimibe therapy. Later, Ali and Martin [182] reported two additional patients with treatment-refractory AA that benefited from treatment with a combination of ezetimibe and simvastatin, in addition to the continuation of intralesional corticosteroid injections. In this report, the putative immunomodulatory effects of statins in relation to the known pathophysiology underlying AA are discussed. Lattouf et al. [183] reported a case series of 29 AA patients with 40–70% scalp involvement treated with simvastatin/ezetimibe 40 mg/10 mg daily. Nineteen completed 24 weeks of treatment, and 14 of 19 were judged responders. Upon completion of the initial 24 weeks of treatment, the responders were randomized into a group of seven patients who continued treatment for an additional 24 weeks, or into a group of seven patients who stopped medication. In the former group, five of seven continued with hair growth or had stable disease, while in the latter group, five of seven patients relapsed. These findings could not be reproduced by subsequent authors who failed to demonstrate efficacy of simvastatin/ezetimibe in 20 [184] respectively 12 patients with AA [185].
Therefore, as a general rule, single case observations or limited case series of successful treatment of a condition like AA, where spontaneous remissions may occur, need to be confirmed by large placebo-controlled trials.
Topical anthralin is a contact irritant that has traditionally been used in the treatment of AA for decades. Unlike DPCP, it creates an irritant versus allergic contact dermatitis of the scalp, and its mode of action in AA is therefore less sophisticated. Nevertheless, small studies have demonstrated results in 25–75% of the patients depending again on the severity of involvement [186, 187]. The treatment is predominantly used in children as an alternative to corticosteroids due to its low risks. A major drawback is the risk of permanent purple discoloration of textiles and bathtubs when used at home.
Topical minoxidil is a hair growth-promoting agent predominantly used for treatment of androgenetic alopecia. In contrast to corticosteroids and immunomodulatory agents, which act on inflammation, minoxidil acts mainly to promote hair growth, and is therefore used in combination with other treatment modalities, such as topical anthralin [188]. Olsen et al. suggested that the use of topical minoxidil may help reducing hair loss after tapering corticosteroids for treatment of AA [189]. It seems reasonable to add topical minoxidil when androgenetic alopecia represents a co-morbidity of AA.
Since cyclosporine (CyS) inhibits the activation of helper T cells that are pathogenic in AA, Gupta et al. [190] originally treated patients with AA with oral CyS 6 mg/kg/day for 12 weeks. Three patients had AU, one had AT and two had patchy AA of the scalp. Hair re-growth in the scalp of all patients occurred between the second and the fourth week of therapy, followed by hair re-growth of the face and body. Overall, the site of best response was the scalp. Cosmetically acceptable terminal hair re-growth on the scalp occurred in three of six patients. Significant hair loss, however, occurred in all patients within 3 months of discontinuation of cyclosporine treatment. Clinical response correlated with changes in immune cell infiltration of the hair follicles. The degree of terminal hair re-growth correlated significantly with decreases in follicular epithelial human lymphocyte antigen-DR and intercellular adhesion molecule-1 expression, T cells, helper/inducer (CD4) T cells, suppressor/cytotoxic (CD8) T cells and Langerhans cells (CD1+DR+) from the hair follicles during cyclosporine therapy. Moreover, hypertrichosis is one of the common side effects of orally administered CyS, encouraging investigators to use the drug in the treatment of AA, but the reports on this subject have been controversial [191]. More recently, combination regimens of oral CyS with low-dose corticosteroids have been found to be effective for treatment of severe AA [192]. Nevertheless, long-term toxicities and risks linked to immunosuppression limit the usefulness of CyS for treatment of AA. Moreover, comparison of IV-MPPT with combination therapy using CyS with low-dose corticosteroid demonstrated that IV-MPPT may be the better treatment option, at least in patients with severe AA of the multipatch type [193].
Other therapies that have not established themselves or lost favour in the treatment of AA include thymopentin [194, 195], isoprinosine [196,197,198], fumaric acid esters [199, 200], phototherapy (PUVA) [201, 202] and hypnotherapy [203].
Finally, platelet-rich plasma (PRP) is blood plasma that has been enriched with platelets. As a concentrated source of autologous platelets, PRP contains and releases through degranulation several growth factors and cytokines. These include platelet-derived growth factor, TGF-beta, fibroblast growth factor, insulin-like growth factors 1 and 2, vascular endothelial growth factor, epidermal growth factor EGF, IL-8 and keratinocyte growth factor KGF. PRP has gained popularity among a limited number of dermatologists with a primary commercial interest, though the use and clinical validation of the method for diverse dermatologic conditions is still in progress. Results of basic science and preclinical trials have yet to be confirmed in large-scale controlled clinical trials. A single, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of PRP on AA has been performed [204]: 45 patients with AA were randomized to receive intralesional injections of PRP, ITA or placebo on one half of their scalp. The other half was not treated. Three treatments were given for each patient, with intervals of 1 month. The end points were hair re-growth, hair dystrophy as measured by dermoscopy, burning or itching sensation and cell proliferation as measured by Ki-67 evaluation. Patients were followed for 1 year. PRP was found to increase hair re-growth significantly and to decrease hair dystrophy and burning or itching sensation compared with ITA or placebo. Ki-67 levels, which served as markers for cell proliferation, were significantly higher with PRP. No side effects were noted during treatment. However, in an era, where a more comprehensive understanding of the immunologic basis of AA has opened the venue to more targeted treatments, the proposal of PRP with its poorly defined mode of action rather represents an intellectual step backwards.
New treatment opportunities based on the results of genome-wide association studies that implicate T cell and natural killer cell activation pathways are paving the way to new approaches in future clinical trials for AA. Currently, there are ongoing studies with the CTLA4-Ig fusion protein abatacept (blocks co-stimulation of T cells), anti-IL15Rβ monoclonal antibodies (block activation of CD8 + T cells) [205] and JAK inhibitors (block signal transduction at the IL-15 receptor) [206, 207].
Craiglow and King [208] originally reported hair growth in a patient with AU treated with the JAK 1 and 3 inhibitor tofacitinib for plaque psoriasis. Xing et al. [209] subsequently reported successful treatment of three patients with AA with the oral JAK 1 and 2 inhibitor ruxolitinib. The patients achieved near-complete hair re-growth within a few months, suggesting the potential clinical utility of JAK inhibition for treatment of AA. Jabbari et al. [210] reported reversal of AA in a patient treated with the oral JAK 1 and 2 inhibitor baracitinib for chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome, which represents yet another condition characterized by prominent IFN signatures. Ultimately, Liu et al. [211] conducted a retrospective study of patients age 18 years or older with AA with at least 40% scalp hair loss treated with tofacitinib. The primary end point was the percent change in the Severity of Alopecia Tool (SALT) score during treatment. Ninety patients met inclusion criteria. Of 65 potential responders to therapy, defined as those with alopecia totalis or alopecia universalis with duration of current episode of disease of 10 years or less or alopecia areata, 77% achieved a clinical response, with 58% of the patients achieving greater than 50% change in SALT score over 4 to 18 months of treatment. Patients with AA experienced a higher percent change in SALT score than did patients with AT/U (81.9 vs 59.0%). Tofacitinib was well tolerated, and there were no serious adverse events. In an attempt to evaluate the benefit and adverse effects of the tofacitinib in a series of adolescent patients with AA, Craiglow et al. [212] reviewed the records of 13 adolescent patients aged 12–17 years with AA treated with tofacitinib. Nine patients experienced clinically significant hair re-growth with a median percent change in SALT score of 93% at an average of 6.5 months of treatment. Adverse events were mild.
Nevertheless, at this time point, affordability of the drug for long-term treatment, sustainability of treatment result [213] and liability of the physician prescribing the drug off label [214] are major obstacles to the treatment of AA with JAK inhibitors, unless patients are enrolled in ongoing clinical studies with either of JAK inhibitors.
In general, any treatment chosen for AA should fulfil the following criteria: remission rates superior to the spontaneous remission rates of AA, proof of efficacy in half side treatment of AT/U and a good safety profile with minimal toxicity. Depending on patient age, surface area, disease duration and co-morbidities, an individual treatment plan can be designed that is successful in a significant proportion of patients (Fig. 4). Figures 5, 6, 7, 8, 9, 10, 11 and 12 illustrate successful treatments of AA following the current lines of therapy. The complexity of its pathogenesis offers opportunities for the development of novel targeted therapies for AA. Ultimately, the options available for adapting to the disease rather than treating it in an effort to cure may also be taken into consideration in selected cases of long-standing or recurrent small spot disease.
Cytotoxic T lymphocyte-associated antigen 4
Ikaros family zinc finger 4
UL16 binding protein
Copy number variants
Melanine concentrating hormone
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy-syndrome
Autoimmune regulator gene
Tumour necrosis factor
Intralesional triamcinolone acetonide
Dual-energy x-ray absorptiometry
Transforming growth factor
Squaric acid dibutyl ester
Severity of Alopecia Tool